Molecular detection of Trypanosoma species and haematological alterations in four trypanosome-infected Nigerian horses
DOI:
https://doi.org/10.15835/nsb13411046Keywords:
anaemia, Nigerian horses, PCR assay, prevalence, Trypanosoma speciesAbstract
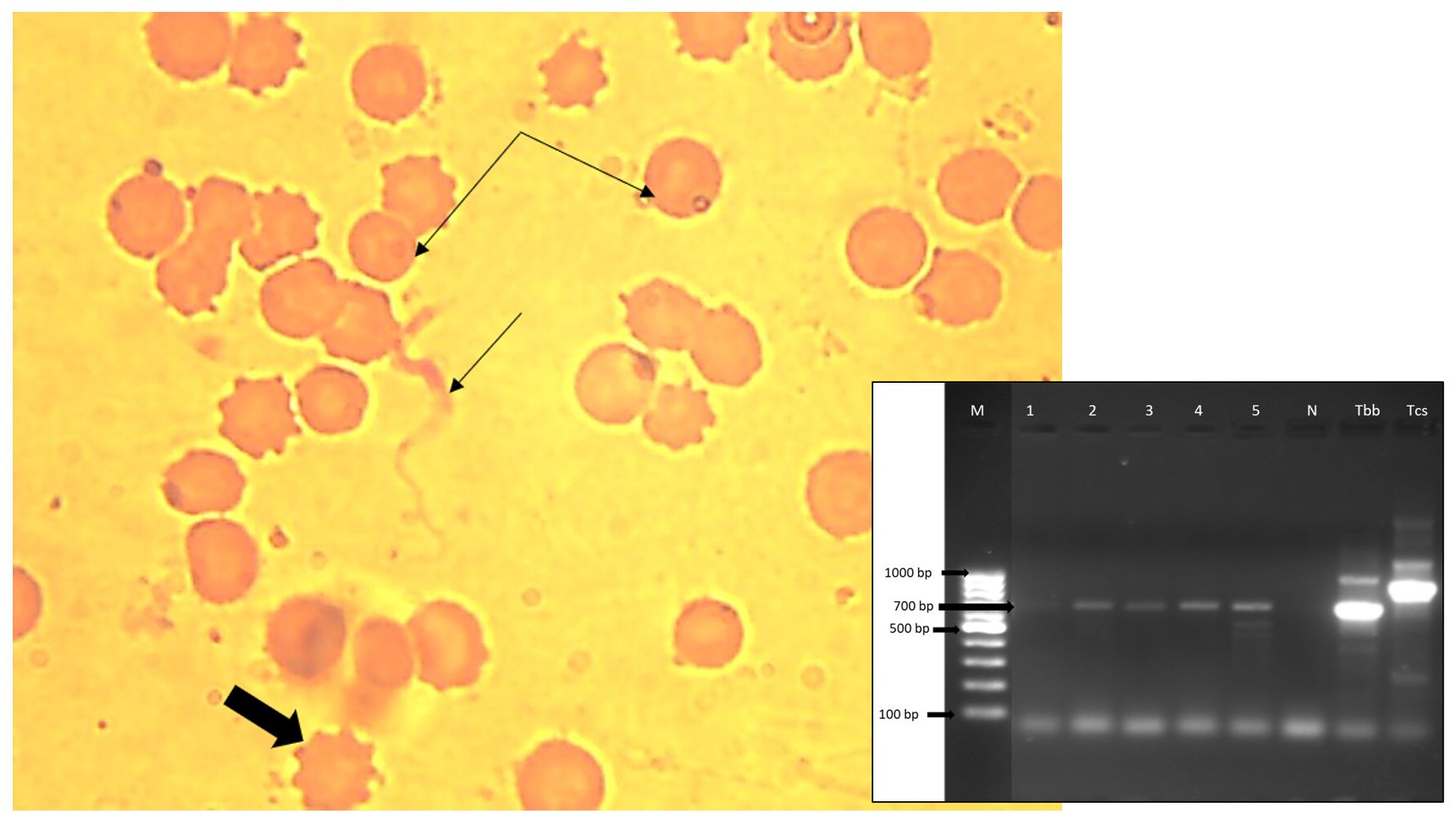
Trypanosomes cause anaemia and are responsible for widespread morbidity and mortality particularly in imported breeds of animals found in sub-tropical and tropical parts of the world. Light microscopy and polymerase chain reaction (PCR) were used to detect trypanosomes in naturally infected Nigerian crossbred horses at Obollo-Afor abattoir, Udenu Local Government Area, Enugu State Nigeria. Blood was collected via the jugular outflow from a total of 200 horses of varying ages and either sex. Conventional procedures were followed during the PCR assay, parasite identification in wet mount, Leishman-stained thin blood and buffy coat smears on glass slides. Light microscopy revealed Trypanosoma species with an elongated, streamlined and tapered body, highly suggestive of T. brucei brucei or its subspecies T. evansi or T. equiperdum. PCR assay produced the expected fragment size of 700 bp specific for ITS-1 region of the 18SrRNA gene of Trypanosoma species in 4 (2%) of 200 blood samples against the routine blood and buffy coat smear examination, which revealed trypanosomes in 3 (1.5%) out of 200 blood samples. Sex and age were not significantly (p>0.05) associated with the trypanosome infection. One of the Trypanosoma infected anaemic horses had microcytic normochromic anaemia, high erythrocyte sedimentation rate and normal leukocyte count, while one of the Trypanosoma species infected non-anaemic horses had erythrocytic parameters and ESR values that are within the reference range, with leukocytosis. It was concluded that the prevalence of equine trypanosomosis was very low, and it’s characterized by mild to moderate anaemia in clinical cases.
Metrics
References
Abbasi HIR, Sahito HA, Sanjrani MI, Abbasi F, Memon MA, Menghwar DR, Kaka NA, Shah MN, Memon M (2014). A disease complex pathogen “trypanosoma congolense” transmitted by tsetse fly in donkeys. Herald Journal of Agriculture and Food Science Research 2(1):44-48.
Adams ER, Malele II, Msangi AR, Gibson WC (2006). Trypanosome identification in wild tsetse populations in Tanzania using generic primers to amplify the ribosomal RNA ITS-1 region. Acta Tropica 100(1-2):103-109. https://doi.org/10.1016/j.actatropica.2006.10.002
Agina OA (2017). Haematology and clinical biochemistry findings associated with equine diseases - a review. Notulae Scientia Biologicae 9(1):1-21. https://doi.org/10.15835/nsb919939
Agina OA, Ihedioha JI (2016). The haematological and serum biochemistry findings associated with natural trypanosome infection in Nigerian horses. In: Proceedings of the 41st Nigerian Society of Animal Production, Ogun State, Nigeria, pp 74-77.
Anosa VO (1988a). Haematological and biochemical changes in human and animal trypanosomiasis. Part I. Revue d’elevage et de Medecine Veterinaire des Pays Tropicaux 41(1):65-78.
Anosa VO (1988b). Haematological and biochemical changes in human and animal trypanosomiasis. Part II. Revue d’elevage et de Medecine Veterinaire des Pays Tropicaux 41(2):151-164.
Auty H, Mundy A, Fyumagwa RD, Picozzi K, Welburn S, Hoare R (2008). Health management of horses under high challenge from trypanosomes: a case study from Serengeti, Tanzania. Veterinary Parasitology 154(3-4):233-241. https://doi.org/10.1016/j.vetpar.2008.02.034
Carnes J, Anupama A, Balmer O, Jackson A, Lewis M, Brown R, … Schnaufer A (2015). Genome and phylogenetic analyses of Trypanosoma evansi reveal extensive similarity to T. brucei and multiple independent origins for dyskinetoplasty. PLoS Neglected Tropical Diseases 9(1):e3404. https://doi.org/10.1371/journal.pntd.0003404
Coles E (1986). Veterinary clinical pathology. WB Saunders Company USA.
Cox AP, Tosas O, Tilley A, Picozzi K, Coleman P, Hide G, Welburn SC (2010). Constraints to estimating the prevalence of trypanosome infections in East African zebu cattle. Parasites and Vectors 3(1):1-8. https://doi.org/10.1186/1756-3305-3-82
Desquesnes M, Dávila AMR (2002). Applications of PCR-based tools for detection and identification of animal trypanosomes: a review and perspectives. Veterinary Parasitology 109(3-4):213-231. https://doi.org/10.1016/s0304-4017(02)00270-4
Desquesnes M, Tresse L (1996). Evaluation of sensitivity of PCR for detecting DNA of Trypanosoma vivax with several methods of blood sample preparations. Revue d’elevage et de Medecine Veterinaire Des Pays Tropicaux 49(4):322-327.
Edwards E (1994). The Encyclopedia of the Horse. Dorling Kindersley.
Ensminger M (1969). Horses and Horsemanship. 4th Ed. The Interstate.
Faye D, Pereira PJL, Almeida D, Goossens B, Osaer S, Ndao M, … Geerts S (2001). Prevalence and incidence of trypanosomosis in horses and donkeys in the Gambia. Veterinary Parasitology 101:101-114. https://doi.org/10.1016/s0304-4017(01)00503-9
Garba UM, Sackey AKB, Agbede RIS, Tekdek LB, Bisalla M (2011). Serum urea and creatinine levels in Nigerian local horses naturally infected with Babesia. Pakistan Veterinary Journal 31(2):163-165.
Hendricks B, Dent A (2007). International Encyclopedia of the Horse (Revised). University of Oklahoma Press.
Higgins TE, Beutler BT, Doumas (2008). Measurement of haemoglobin in blood. In: Burtis CA, Ashwood ER, Bruns D (Eds). Tietz Fundamentals of Clinical Chemistry. Saunders Elsevier USA, pp 524-525.
Hoare C (1972). The trypanosomes of mammals. Blackwell Scientific Publications.
Ihedioha JI, Agina OA (2014). Haematological profile of Nigerian horses in Obollo-Afor, Enugu State. Journal of Veterinary and Applied Sciences 4(1):1-8.
Ihedioha JI, Agina OA (2013). Serum biochemistry profile of Nigerian horses (Equus caballus, Linnaeus 1758). Animal Research International 10(3):1826-1833.
Kihurani DO, Nantulya VM, Mbiuki SM, Mogoa E, Nguhiu-Mwangi J, Mbithi PM (1994). Trypanosoma brucei, T. congolense and T. vivax infections in horses on a farm in Kenya. Tropical Animal Health and Production 26(2):95-101. https://doi.org/10.1007/BF02239908
Lai DH, Hashimi H, Lun ZR, Ayala FJ, Lukes J (2008). Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proceedings of the National Academy of Sciences of the United States of America 105(6):1999-2004. https://doi.org/10.1073/pnas.0711799105
Li FJ, Gasser RB, Lai DH, Claes F, Zhu XQ, Lun, ZR (2007). PCR approach for the detection of Trypanosoma brucei and T. equiperdum and their differentiation from T. evansi based on maxicircle kinetoplast DNA. Molecular and Cellular Probes 21(1):1-7. https://doi.org/10.1016/j.mcp.2006.03.009
Luckins A (1998). Epidemiology of Surra: unanswered questions. Journal of Protozoology Research 8:106-119. https://doi.org/10.32268/jprotozoolres.8.3_106
Lun ZR, Brun R, Gibson W (1992). Kinetoplast DNA and molecular karyotypes of Trypanosoma evansi and Trypanosoma equiperdum from China. Molecular and Biochemical Parasitology 50(2):189-196. https://doi.org/10.1016/0166-6851(92)90215-6
Mijares A, Vivas J, Abad C, Betancourt M, Piñero S, Proverbio F, Marín R, Portillo R (2010). Trypanosoma evansi: Effect of experimental infection on the osmotic fragility, lipid peroxidation and calcium-ATPase activity of rat red blood cells. Experimental Parasitology 124(3):301-305. https://doi.org/10.1016/j.exppara.2009.11.002
Murray M, Murray PK, McIntyre WI (1977). An improved parasitological technique for the diagnosis of African trypanosomiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 71(4):325-326. https://doi.org/10.1016/0035-9203(77)90110-9
Pinchbeck GL, Morrison LJ, Tait A, Langford J, Meehan L, Jallow S, … Christley RM (2008). Trypanosomosis in The Gambia: prevalence in working horses and donkeys detected by whole genome amplification and PCR, and evidence for interactions between trypanosome species. BMC Veterinary Research 4:1-7. https://doi.org/10.1186/1746-6148-4-7
Radostits O, Gay C, Hinchcliff K, Constable P (2007). Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats, and horses. 10th Ed. Elsevier Saunders USA.
Sánchez E, Perrone T, Recchimuzzi G, Cardozo I, Biteau N, Aso P, Mijares A, Baltz T, Berthier D, Balzano-Nogueira L, Gonzatti M (2015). Molecular characterization and classification of Trypanosoma spp. Venezuelan isolates based on microsatellite markers and kinetoplast maxicircle genes. Parasites and Vectors 8(1):1-11. https://doi.org/10.1186/s13071-015-1129-2
Seidl, A, Moraes AS, Aguilar R, Silva MS (1998). A financial analysis of treatment strategies for Trypanosoma evansi in the Brazilian Pantanal. Preventive Veterinary Medicine 33(1-4):219-234. https://doi.org/10.1016/s0167-5877(97)00049-4
Stockham SL, Scott MA (2008). Fundamentals of veterinary clinical pathology. 2nd Ed. Blackwell Publishing USA.
Taylor K, Authie EML (2004). 18 pathogenesis of animal trypanosomiasis. In: Maudlin I, Holmes P, Miles M (Eds). The Trypanosomiases. Cromwell Press.
Thrall MA, Weiser MG (2002). Hematology. In: Hendrix CM (Ed). Laboratory Procedures for Veterinary Technicians (4th ed), Mosby, pp 29-74.
Thrusfield M (2005). Veterinary Epidemiology. 2nd Ed. Blackwell Science Limited.
Ugochukwu E (2009). Animal trypanosomiasis in Africa: aetiology and epidemiology. Animal Research International 5(1). https://doi.org/10.4314/ari.v5i1.48718
Wen Y, Lun Z, Zhu X, Hide G, Lai D (2016). Further evidence from SSCP and ITS DNA sequencing support Trypanosoma evansi and Trypanosoma equiperdum as subspecies or even strains of Trypanosoma brucei. Infection, Genetics and Evolution 41:56-62. https://doi.org/10.1016/j.meegid.2016.03.022
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















