Tetrapleura tetraptera ethylacetate pod extract and its biochemical effects on acetaminophen-induced hepatotoxic female rat
DOI:
https://doi.org/10.55779/nsb14211260Keywords:
antioxidant activity, hepatoprotection, liver damage markers, rats, Tetrapleura tetrapteraAbstract
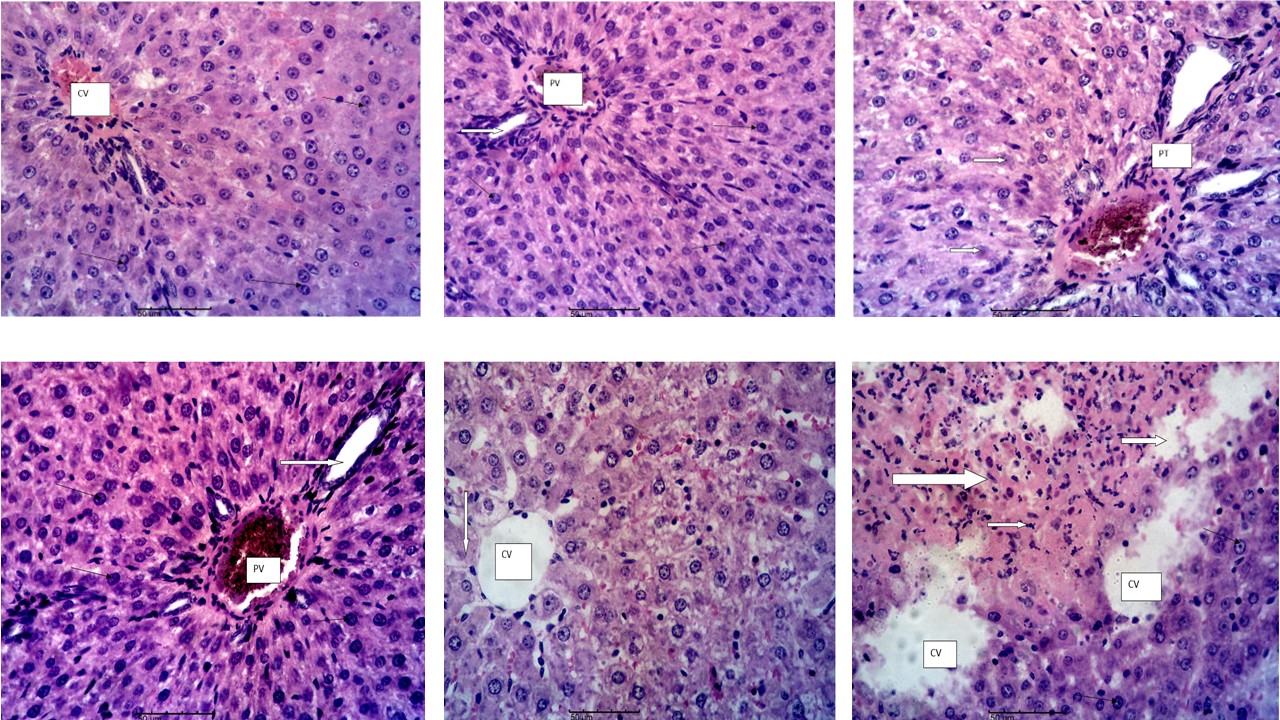
The study investigated the effect of ethyl acetate pod extract of Tetrapleura tetraptera on biochemical markers of liver damage, in vivo and in vitro antioxidant properties using acetaminophen-injured female rats as model. Thirty-five (35) rats assigned into seven groups (A-G) of five rats per group were used for the study. Rats in groups C, D, E and F were pretreated with 100, 200, 400 and 200 mg/kg of the extract while group G rats received Silymarin (100 mg/kg). Distilled water (10 ml/kg) was administered to groups A and B rats. All pretreatments were via the oral route and lasted for seven days. Following pretreatments, acetaminophen (2000 mg/kg) was used to induce liver injury in groups B, C, D, E and G while rats in group A served as normal control. Blood samples were collected 48 h post acetaminophen administration for analyses of the following parameters: Alanine aminotransferase (ALT), alkaline phosphatase (ALP), serum bilirubin, reduced glutathione and superoxide dismutase. Liver tissues were collected for histopathology studies. In vitro antioxidant profile of the extract was evaluated. Results indicated decreases in the activities of ALT and ALP of the extract-treated groups compared to the negative control group. The liver section of the rats treated with 400 mg/kg of the extract showed no lesions and was comparable to that of the normal control rats. The in vivo and in vitro antioxidant activities were in dose and concentration-dependent manner respectively. It was concluded that Tetrapleura tetraptera ethyl acetate extract possesses both hepatoprotective and antioxidant properties.
Metrics
References
Aba PE Ozioko IE, Udem ND, Udem SC (2014). Some biochemical and haematological changes in rats pretreated with aqueous stem bark extract of Lophira Lanceolata and intoxicated with paracetamol (Acetaminophen). Journal of Complementary and Integrative Medicine 11(4):273-277. https://doi.org/10.1515/jcim-2014-0007
Aba PE, Asuzu IU (2018). Mechanisms of actions of some bioactive anti-diabetic principles from phytochemicals of medicinal plants: A review. Indian Journal of Natural Products and Resources 9(2):85-96.
Adesina SK, Iwalewa EO, Johnny II (2016). Tetrapleura tetraptera Taub ethnopharmacology, chemistry, medicinal and nutritional values-a review. British Journal of Pharmaceutical Research 12:1-22. https://doi.org/10.9734/BJPR/2016/26554
Akin-Idowu PE, Ibitoye DO, Ademoyegun OT, Adeniyi OT (2011). Chemical composition of the dry fruit of Tetrapleura tetraptera and its potential impact on human health. Journal of Herbs Spices and Medicinal Plants 17(1):52-61. https://doi.org/10.1080/10496475.2011.560087
Benzie FF, Strain JJ (1999). Ferric reducing/Antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology 299:15-23. https://doi.org/10.1016/s0076-6879(99)99005-5
Beutler E, Duron O, Kelly BM (1963). Improved method for determination of blood glutathione. Journal of Laboratory and Clinical Medicine 61:882-888.
Blay DJ (1997). The distribution and ecological requirements for the growth of Tetrapleura tetra-ptera. Ghana Journal of Forestry 5:40-50.
Boris R, Kuster H, Ugele B, Gruber R, Horn K (2001). Total bilirubin measurement by photometry on a blood gas analyzer: potential for use in neonatal testing at the point of care. Clinical Chemistry 47(10):1845-1847. https://doi.org/10.1093/clinchem/47.10.1845
Burtis CA, Ashwood ER (1999). Tietz textbook of clinical chemistry. Saunders, 3rd edition, pp 336-356.
Colville J (2002). Blood chemistry. In: Hendrix CM (Ed). Laboratory Procedures for Veterinary Technicians. 4th edn. Mosby, St. Louis, pp 75-103.
Doumas BT, Perry BW, Sasse EA, Straumfjord Jr JV (1971) Standardization in bilirubin assays: evaluation of selection methods and stability of bilirubin solutions. Clinical Chemistry 19:984-993.
Drury RA, Wallington A, Cameroun SR (1967). Carlleton’s Histological Techniques. Oxford University Press, New York, pp 1-420.
Erukainure OL, Onifade OF Odjobo B, Olasehinde T, Adesioye T Tugbobo-Amisu O, … Okonrokwo I (2017) Ethanol extract of Tetrapleura tetraptera fruit peels: Chemical characterization, and antioxidant potentials against free radicals and lipid peroxidation in hepatic tissues. Journal of Taibah University for Science 11(6):861-867. https://doi.org/10.1016/j.jtusci.2017.03.007
Fevery J (2008). Bilirubin in clinical practice: A review. Liver International 28:592. https://doi.org/10.1111/j.1478-3231.2008.01716.x
Gill KK, Sandhu HS, Kaurk K (2014). Evaluation of biochemical alterations produced by combined exposure of fenvalerate and nitrate in Bulbalus bubalis. Veterinary World 7(3):146-151.
Jane TN, Matthias OA, Ikechukwu FU (2014). Antioxidant and hepatoprotective activity of fruit extract of Tetrapleura tetraptera. Jordan Journal of Biological Sciences 7(4):251-255.
Johnkennedy N, Adamma E, Austin A, Chukwunyere N (2010). Alterations on biochemical parameters of wister rats administered with sulfadoxine and pyromethamine (Fansidar®). AI Ameen Journal of Medical Sciences 3(4):317-321.
Kakkar SB, Viswanathan P (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry and Biophysics 21:130-132.
Jarsiah P, Karami M, Nosrati A, Alizadeh A, Hashemi-Soteh MB (2019). Circulating miR-122 and miR-192 as specific and sensitive biomarkers for drug-induced liver injury with acetaminophen in rats. Jundishapur Journal of Natural Pharmaceutical Products 14(2):e65678. https://doi.org/10.5812/jjnpp.65678
Kemigisha E, Owusu EO, Elusiyan CA, Omujal F, Tweheyo M Bosu PP (2018). Tetrapleura tetraptera in Ghana, Nigeria and Uganda: house hold uses and local markets. Forests, Trees and Livelihoods. https://doi.org/10.1080/14728028.2018.1498027
Klein B, Read PA, Babson AL (1960). Rapid method for the quantitative determination of serum alkaline phosphatase. Clinical Chemistry 6:269-275.
Laskin DL (1992). Role of macrophages and endothelial cells in hepatotoxicity. In: Billiar TR, Curran RD (Eds). Hepatocyte and Kupffer Cells Interactions. CRC Press, Boca Raton, Florida, pp 147-168.
Mensor F, Luciana L, Fabia S, Menezes G, Leitao AS, Reis TC, … Leitao G (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research 15:127-130. https://doi.org/10.1002/ptr.687
Odesanmi SO, Lawal RA, Ojokuku SA (2009). Effects of ethanolic extract of Tetrapleura tetraptera on liver function profile and histopathology in male Dutch white rabbits. International Journal of Tropical Medicine 4(4):136-139.
Ojewole JAO, Adewunmi CO (2004). Anti-inflammatory and hypoglycaemic effects of Tetrapleura tetraptera (Taub) [Fabaceae] fruit aqueous extract in rats. Journal of Ethnopharmacology 95(2-3):177-182. https://doi.org/10.1016/j.jep.2004.06.026
Okwu DE (2003). The potential of Ocimum gratissimum, Pergularia extensa and Tetrapleura tetraptera as spices and flavouring agents. Nigerian Agriculture Journal 35:143-148.
Pacher P, Beckman JS, Liaudet L (2007). Nitric oxide and peroxynitrite in health and disease. Physiological Reviews 7:315-424. https://doi.org/10.1152/physrev.00029.2006
Panovska TK, Kulevanova S, Gjorgoski I, Bogdanova M, Petrushevska G (2007). Hepatoprotective effect of the ethyl acetate extract of Teucrium polium L. against carbon tetrachloride-induced hepatic injury in rats. Acta Pharmaceutica 57(2):241-248. https://doi.org/10.2478/v10007-007-0020-x
Reitman S, Frankel S (1957). A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. American Journal of Clinical Pathology 28:56-62. https://doi.org/10.1093/ajcp/28.1.56
Sinha AK (1972) Colorimetric assay of catalase. Analytical Biochemistry 47(2):389-394. https://doi.org/10.1016/0003-2697(72)90132-7
Sun, YM, Zhang HY, Chen DZ, Liu CB (2002). Theoretical elucidation on the antioxidant mechanism of curcumin: a DFT study. Organic Letters 4:2909-2911. https://doi.org/10.1021/ol0262789
Sundaram NS, Hemshekhar M, Thushara RM, Santhosh MS, Kumar SK, Paul M, … Girish KS (2014). Tamarind seed extract mitigates the liver oxidative stress in arthritic rats. Food and Function 5:587-597. https://doi.org/10.1039/c3fo60381d
Svegliati-Baroni G, Saccomanno S, van Goor H, Jansen P, Benedetti A, Svegliati-Baroni G, Moshage H (2001) Involvement of reactive oxygen species and nitric radicals in activation and proliferation of rat hepatic stellate cells. Liver 21:1-12. https://doi.org/10.1034/j.1600-0676.2001.210101.x
Vermeulen NP, Bessems JG, Van de Streat R (1992). Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism-based prevention. Drug Metabolism Review 24:13. https://doi.org/10.3109/03602539208996298
Yuan L, Kaplowitz N (2008). Glutathione in liver diseases and hepatotoxicity. Molecular Aspects of Medicine 30(1-2):29-41. https://doi.org/10.1016/j.mam.2008.08.003
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















