GC-MS analysis and antioxidant potential of wild underutilized medicinally important legume, velvet bean (Mucuna pruriens L. DC.)
DOI:
https://doi.org/10.15835/nsb14111098Keywords:
antioxidants, GC-MS, Mucuna pruriens, phytochemicalsAbstract
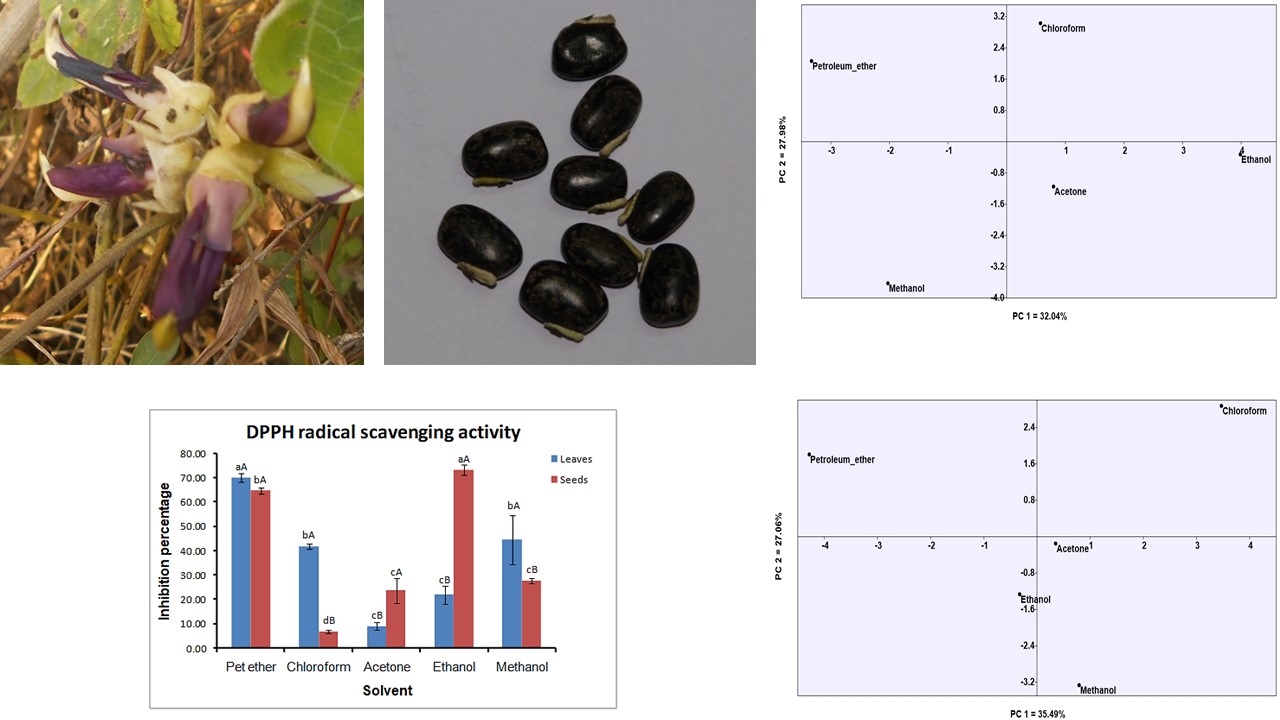
Mucuna pruriens (L). DC is one of the most promising wild underutilized medicinal legume belonging to family Fabaceae. It is used in ayurvedic as well as various traditional systems of medicine. This plant was widely utilized in treatment of various disorders. Also, it is a rich source of nutrients as well as used as a flavouring agent in bakery industry. The present study was aimed to investigate leaves and seeds antioxidant potential by DPPH assay and phytochemicals by preliminary phytochemical screening and Gas Chromatography-Mass Spectroscopy (GC-MS) analysis in five different solvents. Highest antioxidant activity was found to be 76.96% in seeds extracted with ethanol and 72.50% in leaves extracted with petroleum ether. While preliminary phytochemical screening revealed presence of alkaloids, flavonoids, phenols, tannins, saponins, glycosides, steroids and terpenoids. GC-MS analysis revealed twenty-four and thirty bioactive compounds from the leaves and seeds respectively and it was solvent specific. Antioxidant, antifungal, antimicrobial, anti-malarial, anti-diabetic, anti-cancerous, and hypocholesterolemic properties have been reported to compounds which were found in present study. However, reported bioactive compounds highlight its nutritional importance and validate the use of the plant to cure various disorders by traditional practitioners. While the antioxidant potential and phytochemical investigations will direct their potential for utilization and applicability as a nutraceutical.
Metrics
References
Airaodion AI, Olatoyinbo PO, Ogbuagu U, Ogbuagu EO, Akinmolayan JD, Adekale OA, Airaodion EO (2019). Comparative assessment of phytochemical content and antioxidant potential of Azadirachta indica and Parquetina nigrescens leaves. Asian Plant Research Journal 1-14. https://doi.org/10.9734/aprj/2019/v2i330045
Akpuaka A, Ekwenchi MM, Dashak DA, Dildar A (2013). Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Natural Sciences 11:141-147.
Andiappan L, Rajendran S, Vediappen P (2017). Synthesis and biological evaluation of new benzofuran carboxamide derivatives. Journal of Saudi Chemical Society 21:277-285. https://doi.org/10.1016/j.jscs.2015.06.008
Bagavathi PE, Ramasamy N (2012). GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacognosy Research 1(4):11-14. https://doi.org/10.4103/0974-8490.91028
Balachandran C, Lakshmi RS, Duraipandiyan V, Ignacimuthu S (2012). Antimicrobial activity of Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil from Chennai, India. Bioresource Technology 129.
Bashir A, Ibrar K, Shumaila B, Sadiq A (2012). Chemical composition and antifungal, phytotoxic, brine shrimp cytotoxicity, insecticidal, and antibacterial activities of the essential oils of Acacia modesta. Journal of Medicinal Plants Research 6:4653-4659. https://doi.org/10.5897/JMPR12.016
Bhusare BP, Ahire ML, John CK, Nikam TD (2021a). Uraria picta: A comprehensive review on evidences of utilization and strategies of conservation. Journal of Phytology 13:41-47. https://doi.org/10.25081/jp.2021.v13.7028
Bhusare BP, John CK, Bhatt VP, Nikam TD (2021b). Colchicine induces tetraploids in in vitro cultures of Digitalis lanata Ehrh.: Enhanced production of biomass and cardiac glycosides. Industrial Crops and Products 174:114167. https://doi.org/10.1016/j.indcrop.2021.114167
Bhusare BP, John CK, Bhatt VP, Nikam TD (2018) In vitro propagation of Digitalis lanata Ehrh. through direct shoot regeneration A source of cardiotonic glycosides. Industrial crops and products 121:313-319. https://doi.org/10.1016/j.indcrop.2018.05.019
Casagrande M, Zanela J, Júnior AW, Busso C, Wouk J, Iurckevicz G, Malfatti CRM (2018). Influence of time, temperature and solvent on the extraction of bioactive compounds of Baccharis dracunculifolia: In vitro antioxidant activity, antimicrobial potential, and phenolic compound quantification. Industrial Crops and Products 125:207-219. https://doi.org/10.1016/j.indcrop.2018.08.088
Chandrasekaran M, Senthilkumar A, Venkatesalu V (2011). Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. European Review for Medical and Pharmacological Sciences 15:775-780.
Dandekar R, Fegade B, Bhaskar VH (2015). GC-MS analysis of phytoconstituents in alcohol extract of Epiphyllum oxypetalum leaves. Journal of Pharmacognosy and Phytochemistry 4:149-154.
Devhade JB, Kalwaghe SS, Devade MJ (2015). Mucuna nivea DC. A member of Fabaceae: Preliminary phytochemical analysis. The Pharma Innovation Journal 4:96-98.
Dhawan D, Gupta J (2017). Research article comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. International Journal of Biological Chemistry 11(1):17-22. https://doi.org/10.3923/ijbc.2017.17.22
Dhore MA (1986). Flora of Amravati District With special reference to the distribution of free species, PH. D Thesis, PUB (2002) Amravati University, Amravati.
Duke’s (2013). Phytochemical and ethno botanical databases, phytochemical and ethnobotanical databases. www.ars-gov/cgi-bin/duke/
Duguma HT (2020). Wild edible plant nutritional contribution and consumer perception in Ethiopia. International Journal of Food Science 2958623:16. https://doi.org/10.1155/2020/2958623
Edeoga HO, Okwu DE, Mbaebie BO (2005). Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology 4:685-688. https://doi.org/10.5897/AJB2005.000-3127
Faridha Begum I, Mohankumar R, Jeevan M, Ramani K (2016). GC-MS Analysis of bio-active molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian Journal of Microbiology 56:426-432. https://doi.org/10.1007/s12088-016-0609-1
Ganesh M, Mohankumar M (2017). Extraction and identification of bioactive components in Sida cordata (Burm. f.) using gas chromatography-mass spectrometry. Journal of Food Science and Technology 54:3082-3091. https://doi.org/10.1007/s13197-017-2744-z
Golubović T, Palić R, Kitić D, Stojanović G, Zlatković B, Ristić M, Pavlović D (2014). Composition, antioxidant and antimicrobial activities of methanol extracts of some Acinos miller species. Natural Product Communications 9:731-735. https://doi.org/10.15835/nbha48111782
Gopalkrishanan S, Vadivel E (2011). GC-MS analysis of some bioactive constituents of Mussaenda frondosa L. International Journal of Pharma and Bio Sciences 2:313-320.
Harborne JB (1998). Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 1st edn. Chapman and Hall; London, UK.
Hema R, Kumaravel S, Alagusundaram (2011). GC/MS determination of bioactive components of Murraya koenigii. Journal of American Science 7:1.
Hooker JD (1875). The flora of British India. Nature 12:3-5. https://doi.org/10.1038/012003a0
Jahanban-Esfahlan A, Ostadrahimi A, Tabibiazar M, Amarowicz R (2019). A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules 24(11):2133. https://doi.org/10.3390/molecules24112133
Kabera JN, Semana E, Mussa AR, He X (2014). Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. Journal of Pharmacy and Pharmacology 2(7):377-392.
Kalaivani MK, Bhavana J, Sumathy A (2013). GC-MS analysis of chloroform extract of Croton bonplandianum. International Journal of Pharma and Bio Sciences 4:613-617.
Kasote DM, Katyare SS, Hegde MV, Bae H (2015). Significance of antioxidant potential of plants and its relevance to therapeutic applications. International Journal of Biological Sciences 11(8):982. https://doi.org/10.7150/ijbs.12096
Kehrer JP, Klotz LO (2015). Free radicals and related reactive species as mediators of tissue injury and disease: implications for health. Critical Reviews in Toxicology 45(9):765-798. https://doi.org/10.3109/10408444.2015.1074159
Lampariello LR, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G (2012). The magic velvet bean of Mucuna pruriens. Journal of Traditional and Complementary Medicine 2(4):331-339. https://doi.org/10.1016/s2225-4110(16)30119-5
Longhi JG, Perez E, José de Lima J, Cândido LMB (2011). In vitro evaluation of Mucuna pruriens (L.) DC. antioxidant activity. Brazilian Journal of Pharmaceutical Sciences 47:3. https://doi.org/10.1590/S1984-82502011000300011
Mankar GD, Wayase UR, Shelke DB, Raskar KB, Nikam TD, Barmukh RB (2021b). Time-dependent determinative biochemical traits for salt tolerance mechanism in mungbean (Vigna radiata (L.) R. Wilczek). Journal of Experimental Biology and Agricultural Sciences 9(2):152-171. http://dx.doi.org/10.18006/2021.9(2).152.171
Mannaa M, Kim KD (2018). Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: Study II. Mycobiology 46:52-63. https://doi.org/10.1080/12298093.2018.1454015
Milanović Z, Tošović J, Marković S, Marković Z (2020). Comparison of the scavenging capacities of phloroglucinol and 2,4,6-trihydroxypyridine towards HO˙ radical: a computational study. RSC Advance 10:43262-43272. https://doi.org/10.1039/D0RA08377A
Mishra L, Wagner H (2006). Lipid derivatives from Mucuna pruriens seeds. Indian Journal of Chemistry 45: 801-804.
Moreira DDL, Teixeira SS, Monteiro MHD, De-Oliveira ACA, Paumgartten FJ (2014). Traditional use and safety of herbal medicines. Revista Brasileira de Farmacognosia 24:248-257. https://doi.org/10.1016/j.bjp.2014.03.006
Nikalje GC, Shelke DB, Yadav K, Suprasanna P (2019). Halophytes: prospective plants for future. Hasanuzzaman M et al. (Eds). Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes. https://doi.org/10.1007/978-981-13-3762-8_10
Nurettin Y, Canan G, Osman U, Ahmet Y, Serdar U, Kamil C, Salih T (2006). Composition and antimicrobial activities of volatile components of Minuaritia meyeri. Turkish Journal of Chemistry 30:70-76.
Olayinka OA, Jessica TI, Taiwo FO, Anuoluwa AA (2015). Exploration of the chemistry and biological properties of pyrimidine as a privilege pharmacophore in therapeutics. International Journal of Biological Chemistry 9:148-177. https://doi.org/10.3923/ijbc.2015
Peach K, Tracy MV (1955). Modern Methods of Plant Analysis. Springer-Verlag, Heidelberg.
Phaniendra A, Jestadi DB, Periyasamy L (2015). Free radicals: properties, sources, targets, and their implication in various diseases. Indian journal of clinical biochemistry 30(1):11-26. https://doi.org/10.1007/s12291-014-0446-0
Philomena G (2011). Concerns regarding the safety and toxicity of medicinal plants an overview. Journal of Applied Pharmaceutical Science 1:40-44.
Pietro Z, Maurizio S, Maurizio B, Antonella M, Sergio R, Carmen F, Felice S (2010). Essential oil composition of stems and fruits of Caralluma europaea N.E.Br. (Apocynaceae). Molecules 15:627-638. https://doi.org/10.3390/molecules15020627
Revathi P, Jeyaseelansenthinath T, Thirumalaikolundhusubramaian P (2014). Preliminary phytochemical screening and GC-MS analysis of ethanolic extract of mangrove plant-Bruguiera cylindrica (rhizho) L. International Journal of Pharmacognosy and Phytochemical Research 6:729-740.
Saczewski F, Balewski Ł (2009). Biological activities of guanidine compounds. Expert Opinion on Therapeutic Patents 19:1417-1448. https://doi.org/10.1517/13543770903216675
Sahoo N, Manchikant P (2013). Herbal drug regulation and commercialization: an Indian industry perspective. Journal of Alternative and Complementary Medicine 19:957-963. https://doi.org/10.1089/acm.2012.0275
Sallam KI (2007). Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 18:566-575. https://doi.org/10.1016/j.foodcont.2006.02.002
Savithramma N, LingaRao M, Suhrulatha D (2011). Screening of medicinal plants for secondary metabolites. Middle-East Journal of Scientific Research 8:579-584.
Sermakkani M, Thangarpandia V (2012). GC-MS analysis of Cassia italica leaf methanol extract. Asian Journal of Pharmaceutical and Clinical Research 5:90-94.
Shanmugavel G, Krishnamoorthy G (2018). Nutraceutical and phytochemical investigation of Mucuna pruriens seed. The Pharma Innovation Journal 7:273-278.
Shelke DB, Pandey M, Nikalje GC, Zaware BN, Suprasanna P, Nikam TD (2017). Salt responsive physiological, photosynthetic and biochemical attributes at early seedling stage for screening soybean genotypes. Plant Physiology and Biochemistry 118:519-528. https://doi./org/10.1016/j.plaphy.2017.07.013
Simmons AD (2018). Parkinson’s Disease. Rakel D (Ed). Integrative Medicine (Fourth Edition). Elsevier, pp 143-151. https://doi.org/10.1016/B978-0-323-35868-2.00015-3
Sonawane H, Ghule S, Math S, Shelke D, Nikalje G (2021). Rhizoctonia bataticola: From plant pathogen to a potential source of pharmaceutically relevant metabolites. Current Research in Green and Sustainable Chemistry 4:100171. https://doi.org/10.1016/j.crgsc.2021.100171
Syeda FA, Habib-Ur R, Choudahry MI, Atta-Ur-Rahman (2011). Gas chromatography-mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bioassays of crude extract of Iris germanica. International Journal of Genetics and Molecular Biology 3:95-100.
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011). Phytochemical screening and extraction: A Review. Internationale Pharmaceutica Sciencia 1:98-106.
Tripathi YB, Upadhyay AK (2002). Effect of the alcohol extract of the seeds of Mucuna pruriens on free radicals and oxidative stress in albino rats. Phytotherapy Research 16:534-538. https://doi.org/10.1002/ptr.962
Umdale S, Ahire M, Aiwale V, Jadhav A, Mandada P (2020). Phytochemical investigation and antioxidant efficacy of wild, underutilized berries of economically important Indian Sandalwood (Santalum album L.). Biocatalysis and Agricultural Biotechnology 27:101705. https://doi.org/10.1016/j.bcab.2020.101705
Umdale S, Mahadik R, Otari P, Gore N, Mundad P, Ahire M (2021). Phytochemical composition, and antioxidant potential of Frerea indica Dalz.: A critically endangered, endemic and monotypic genus of the Western Ghats of India. Biocatalysis and Agricultural Biotechnology 35:102060. https://doi.org/10.1016/j.bcab.2021.102080
Venkata Raman B, La S, Pardha SM, Narashimha Rao B, Naga Vamsi Krishna A, Sudhakar M, Radhakrishnan TM (2012). Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian Journal of Pharmaceutical and Clinical Research 5:99-106.
Verpoorte R (1998). Exploration of nature’s chemo diversity: the role of secondary metabolites as leads in drug development. Drug Discovery Today 3:232-238.
Von Gadow A, Joubert E, Hansmann CF (1997). Comparison of antioxidant activity of aspalathin with that of other plant phenols of Rooibosed tea (Aspalathonlinearis), α-tocopherol, BHT and BHA. Journal of Agricultural and Food Chemistry 45:632-638. https://doi.org/10.1021/jf960281n
Yen GC, Duh PD (1994). Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. Journal of Agricultural and Food Chemistry 42:629-632. https://doi.org/10.1021/jf00039a005
Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J (2012). Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Global Journal of Pharmacology 6:65-71.
Zakaria NA, Ibrahim D, Shaida SF, Supardy NA (2011). Phytochemical Composition and Antibacterial Potential of Hexane Extract from Malaysian Red Algae, Acanthophora spicifera (Vahl) Borgesen. World Applied Sciences Journal 15:496-501.
Zia-Ul-Haq M, Shahid SA, Ahmad S, Qayum M, Khan I (2012). Antioxidant potential of various parts of Ferula assafoetida L. Journal of Medicinal Plants Research 6(16):3254-3258. https://doi.org/10.5897/JMPR12.141
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















