Cytogenetic, chromosome count optimization and automation of Neolamarckia cadamba (Rubiaceae) root tips derived from in vitro mutagenesis
DOI:
https://doi.org/10.15835/nsb13310995Keywords:
ImageJ, mitotic chromosomes, polyploid, Rubiaceae, single and double stainingAbstract
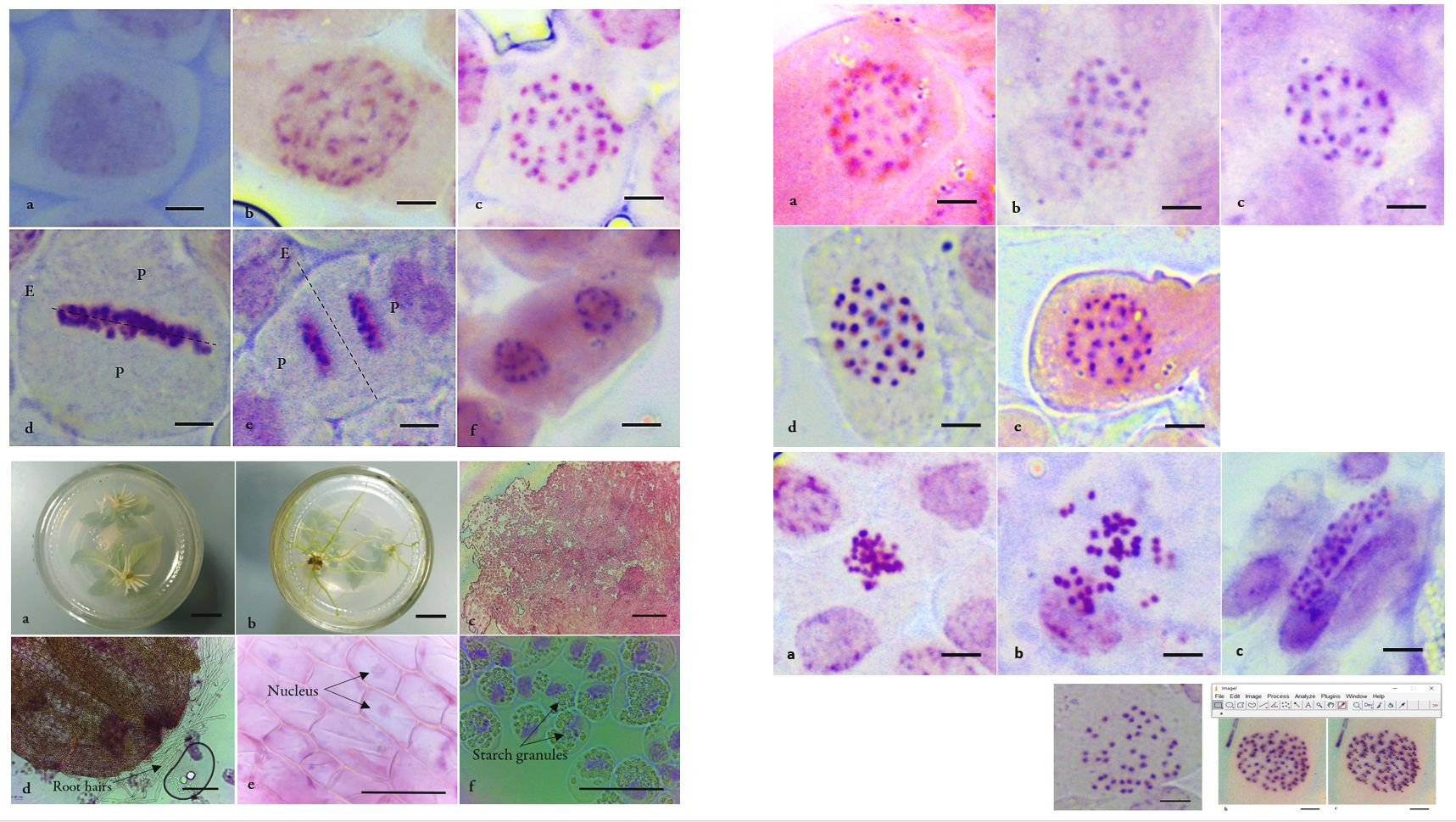
Chromosome count is the only direct way to determine the number of chromosomes of a species. This study is often considered trivial that seldom described and discussed in detail. Therefore, it is inevitable that the chromosome count protocol should be revised and revisited before it becomes obliterated. In the present study, we encountered challenges in obtaining a clear micrograph for the chromosome count of active mitotic cells of Neolamarckia cadamba (Roxb.) Bosser (Rubiaceae) root tips. Several obstacles were determined through micrograph observation, such as existing unwanted particles in cells, poor chromosome staining and chromosome clumping. To overcome these, root tip types, staining methodologies, squashing methods were among the factors assessed to obtain clear micrographs. The chromosome counts of N. cadamba under optimized procedure showed 2n = 44 chromosomes. We also apply digital technology in chromosome counts, such as online databases and graphic software that are open source and freely accessible to the public. Only basic laboratory equipment and chemicals were used throughout the study, thus making this study economical and applicable in a basic laboratory. The availability of online digital software and databases provide open-source platforms that will ease the efforts in chromosome count.
Metrics
References
Barbat C, Rodino S, Petrache P, Butu M, Butnariu M (2013). Microencapsulation of the allelochemical compounds and study of their release from different products. Digest Journal of Nanomaterials and Biostructures 8(3):945-953. https://www.chalcogen.ro/945_Butu.pdf
Bedi YS, Bir SS, Gill BS (1981). Cytopalynology of woody taxa of family Rubiaceae from North and Central India. Proceeding Indian Natural Science Academy B47(6):708-715. https://insa.nic.in/writereaddata/UpLoadedFiles/PINSA/Vol47B_1981_5_Art16.pdf
Bedini G, Peruzzi L (2015). A comparison of plant chromosome number variation among Corsica, Sardinia and Sicily, the three largest Mediterranean islands. Caryologia 68(4):289-293. https://doi.org/10.1080/00087114.2015.1109932
Bosser J (1984). Bulletin du Muséum National d'Histoire Naturelle Section B, Adansonia, botanique, phytochimie. Muséum, Paris. https://www.biodiversitylibrary.org/bibliography/13855
Bostan C, Butnariu M, Butu M, Ortan A, Butu A, Rodino S, Parvu C (2013). Allelopathic effect of Festuca rubra on perennial grasses. Romanian Biotechnological Letters 18(2):8190-8196. https://e-repository.org/rbl/vol.18/iss.2/14.pdf
Candan F (2013). Some observations on plant karyology and investigation methods. Current Protocol Biology Research 217-55. https://doi.org/10.5772/56081
Corréa AM, Forni-Martins ER (2004). Chromosomal studies of species of Rubiaceae (A. L. de Jussieu) from the Brazilian cerrado. Caryologia 57(3):250-258. https://doi.org/10.1080/00087114.2004.10589400
de Alencar, LD, Azevedo P, Latado RR (2020). Mothers’ command: phenotypes changes resulting from reciprocal interploidy crosses. Euphytica 216:21. https://doi.org/10.1007/s10681-020-2557-4
Eng WH, Ho WS, Ling KH (2021). Effects of colchicine treatment on morphological variations of Neolamarckia cadamba. International Journal of Agricultural Technology 17(1):47-66. http://www.ijat-aatsea.com/past_v17_n1.html
Gujendran V, Rodriguez JJ (2004). Chromosome counting via digital image analysis. International Conference on Image Processing 2929-2932. https://doi.org/10.1109/ICIP.2004.1421726
Ho WS, Pang SL, Julaihi A (2014). Identification and analysis of expressed sequence tags present in xylem tissues of kelampayan (Neolamarckia cadamba (Roxb.) Bosser). Physiology and Molecular Biology – Plants 20(3):393-397. https://doi.org/10.1007/s12298-014-0230-x
Huang H (2016). Systematics and genetic variation of Actinidia. Academic Press, United States. https://doi.org/10.1016/B978-0-12-803066-0.00001-0
Ianculov I, Palicica R, Butnariu M, Dumbrava D, Gergen I (2005). Achieving the crystalline state of chlorophyll of the Fir–tree (Abies alba) and the pine (Pinus ylvestris). Revista de Chimie 56(4):441-443. https://ad-astra.ro/2016/05/05/the-obtaining-of-chlorophyll-in-crystalline-form-from-fir-needles-abies-alba-and-from-pine-needles-pinus-silvestris/
Jiang Y, Liu S, Hu J, He G, Liu Y, Chen X, … Gao S (2020). Polyploidization of Plumbago auriculata Lam. in vitro and its characterization including cold tolerance. Plant Cell, Tissue and Organ Culture 140:315-325. https://doi.org/10.1007/s11240-019-01729-w
Khoshoo TN, Sc FA, Bhatia SK (1963). Cytology of some Rubiaceae of the North-western Himalayas. Proceedings of the Indian Academy of Sciences 58(B):36-44. https://doi.org/10.1007/BF03052070
Kiehn M (1986). Karyosystematic studies on Rubiaceae: Chromosome counts from Sri Lanka. Plant Systematics and Evolution 154:213-23. https://doi.org/10.1007/BF00990124
Kiehn M (1995). Chromosome survey of the Rubiaceae. Annals of the Missouri Botanical Garden 82(3):398-408. https://doi.org/10.2307/2399890
Kiehn M (2010). Chromosomes of neotropical Rubiaceae. I: Rubioideae. Annals of the Missouri Botanical Garden 97(1):91-105. https://doi.org/10.3417/2007115
Kiehn M, Lorence DH (1996). Chromosome counts on Angiosperms cultivated at the National Tropical Botanical Garden, Kauai, Hawaii. Pacific Science 50(3):317-323. http://hdl.handle.net/10125/2903
Kim JS, Seo MS, Moon MS, Won SY, Kwon SJ (2019). Insight on doubled haploid production with an amphidiploid species ‘Dolsangat’ in Brassica juncea. Korean Journal Breeding Science 51(4):341-350. https://doi.org/10.9787/KJBS.2019.51.4.341
Lavania UC (2020). Plant speciation and polyploidy: in habitat divergence and environmental perspective. Nucleus 63:1-5. https://doi.org/10.1007/s13237-020-00311-6
Lee YS (1979). Remarks on chromosome number in Rubiaceae. Korean Journal of Plant Taxon 9(1):57-66. https://doi.org/10.11110/kjpt.1979.9.1.057
Maluszynska J (2003). Cytogenetics test for ploidy level analyse-chromosome counting. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (Eds). Doubled haploid production in crop plants. Springer, Netherland pp 391-395. https://doi.org/10.1007/978-94-017-1293-4
Martin C, Viruel MA, Lora J, Hormaza JI (2019). Polyploidy in fruit tree crops of the Genus Annona (Annonaceae). Frontiers in Plant Science 10:99. https://doi.org/10.3389/fpls.2019.00099
Mathew PM, Philip O (1986). The distribution and systematic significance of pollen nuclear number in the Rubiaceae. Cytologia 51:117-124. https://doi.org/10.1508/cytologia.51.117
Matsushima R (2015). Morphological variations of starch grains. In: Nakamura Y (Ed). Starch. Springer, Japan pp 425-441. https://doi.org/10.1007/978-4-431-55495-0_13
Mehra PN, Bawa KS (1969). Chromosomal evolution in tropical hardwoods. Evolution 23(3):466-481. https://doi.org/10.2307/2406701
Mehrvarz SS, Ghadim SM (2015). Chromosome counts of some species of the genus Galium (Rubiaceae) from Iran. Feddes Repertorium 126:31-36. https://doi.org/10.1002/fedr.201400026
Midin MR, Nordin MS, Madon M, Saleh MN, Goh H, Noor NM (2018). Determination of the chromosome number and genome size of Garcinia mangostana L. via cytogenetics, flow cytometry and k-mer analyses. Caryologia 1017:1-10. https://doi.org/10.1080/00087114.2017.1403762
Ochatt SJ, Patat-Ochatt EM, Moessner A (2011). Ploidy level determination within the context of in vitro breeding. Plant Cell, Tissue and Organ Culture 104:329-341. https://doi.org/10.1007/s11240-011-9918-6
Pang SL, Ho WS, Mat-Isa MN, Julaihi A (2015). Gene discovery in the developing xylem tissue of a tropical timber tree species: Neolamarckia cadamba (Roxb.) Bosser (Kelampayan). Tree Genetics & Genomes 11:47. https://doi.org/10.1007/s11295-015-0873-y
Pfister B, Zeeman SC (2016). Formation of starch in plant cells. Cellular and Molecular Life Sciences 73:2781-807. https://doi.org/10.1007/s00018-016-2250
Puangsomlee P, Puff C (2001). Chromosome numbers of Thai Rubiaceae. Nordic Journal of Botany 21(2):165-176. https://doi.org/10.1111/j.1756-1051.2001.tb01354.x
Rice A, Šmarda P, Novosolov M, Drori M, Glick L, Sabath N, Meiri S, Belmaker J, Mayrose I (2019). The global biogeography of polyploid plants. Nature Ecology and Evolution 3:265-273. https://doi.org/10.1038/s41559-018-0787-9
Rivero R, Emily B, Sessa EB, Zenil-Ferguson R (2019). EyeChrom and CCDBcurator: Visualizing chromosome count data from plants. Application in Plant Sciences 7(1):e1207. https://doi.org/10.1002/aps3.1207
Selvaraj R (1987). Karyomorphological studies in South Indian Rubiaceae. Cytologia 52:343-56. https://doi.org/10.1508/cytologia.52.343
Sharma A, Sen S (2019). Chromosome botany. CRC Press, Boca Raton. https://doi.org/10.1201/9780429187612
Sharma AK, Sharma A (1980). Chromosome techniques theory and practice. Butterworth & Co. Ltd., United Kingdom. https://doi.org/10.1016/C2013-0-01036-5
Singh RJ (2016). Plant cytogenetics. CRC Press, USA. https://doi.org/10.1201/9781315374611
Singh RJ (2017). Practical manual on plant cytogenetics. CRC Press, Boca Raton. https://doi.org/10.4324/9781351228268
Tchin BL, Ho WS, Pang SL (2018a). Isolation and characterisation of cinnamate 4-hydroxylase (C4H) gene controlling the early stage of phenylpropanoid biosynthetic pathway in developing xylem tissues of kelampayan (Neolamarckia cadamba, Rubiaceae). Asian Journal of Agriculture and Biology 6(2):278-286. https://www.asianjab.com/wp-content/uploads/2018/06/26.-OK_Isolation-and-in-silico-characterization-of-cinnamate-4-hydroxylase-c4h-gene-controlling-the-early1.pdf
Tchin BL, Ho WS, Pang SL (2018b). Isolation and in silico characterization of full-length cinnamyl alcohol dehydrogenase gene involved in lignin biosynthesis in Neolamarckia cadamba. Journal of Applied Biology and Biotechnology 6(2):1-5. http://doi.org/10.7324/JABB.2018.60201
Windham MD, Pryer KM, Poindexter DB, Li F, Rothfels CJ, Beck JB (2020). A step-by-step protocol for meiotic chromosome counts in flowering plants: A powerful and economical technique revisited. Applications in Plant Sciences 8(4):e11342. https://doi.org/10.1002/aps3.11342
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















