Record on dominant microfungi and their potential phosphate solubilization in tea garden soils
DOI:
https://doi.org/10.15835/nsb14110958Keywords:
Aspergillus, microfungi, Mokokchung, Penicillium, tea gardens, TrichodermaAbstract
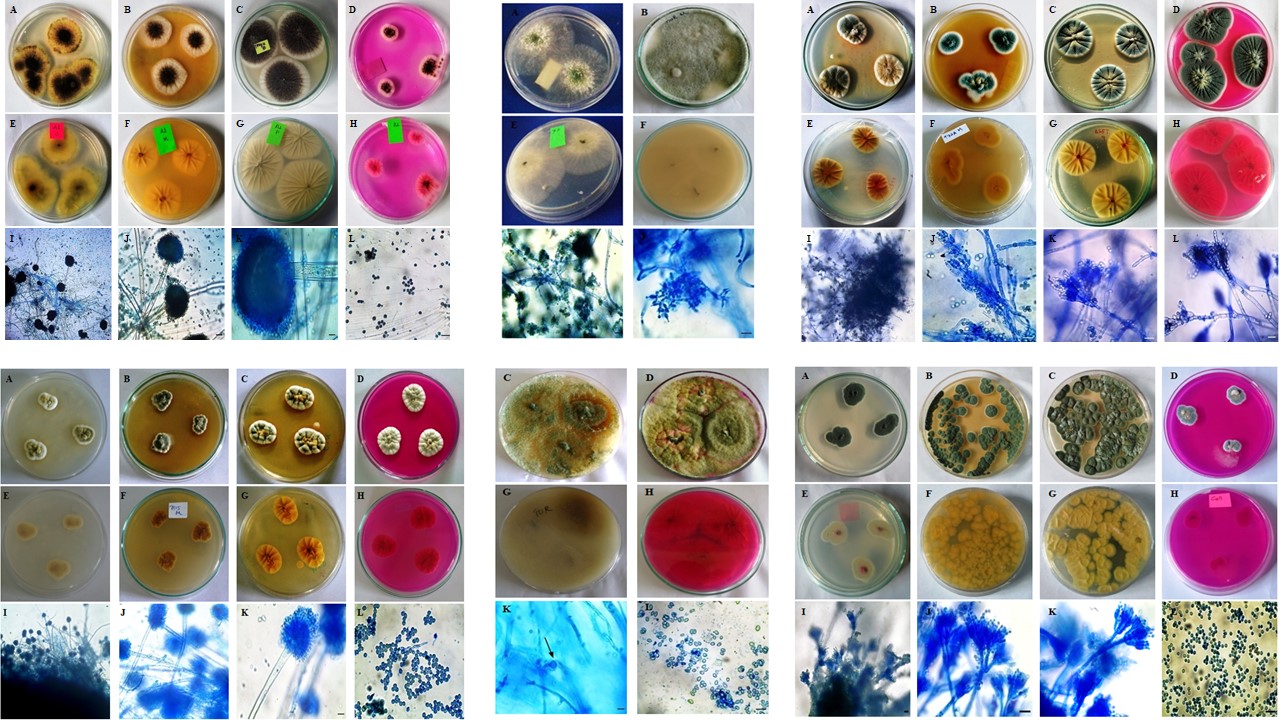
Microfungi are one of the important microbial groups in agriculture due to their positive, mutualistic and negative effect on plant growth and productivity. The roles of fungi extend from organic matter decomposition and mineral cycling to plant growth promotion. Considering these indispensable roles of this microbial group, the present research was undertaken to investigate the indigenous dominant microfungi in tea garden soils of Mokokchung district, Nagaland in India. The dominant microfungi were screened for their phosphate solubilization activity in PVK agar medium using tri-calcium phosphate as the sole phosphate source. Microfungal isolates showed significant differences in culture plates as well as microscopic studies. A total of 110 fungal isolates under 19 genera were identified in the present study. Among the soil microfungi, Aspergillus, Penicillium and Trichoderma were found to dominate the studied tea garden soils. The highest phosphate solubilization activities were observed for species under Aspergillus (1.95 cm to 1.71 cm) followed by Penicillium (1.57 cm to 1.18 cm) and Trichoderma species (1.13 cm to1.06 cm). The present study offers a glimpse of indigenous microfungi as well as provide information on the dominant microfungi in tea garden soils of Mokokchung district, Nagaland and hence, will aid and expand knowledge on indigenous fungi and their various roles. Also, the applications of potent phosphate-solubilizers isolated in this study can be a future source of biofertilizers consortium for tea and other plants.
Metrics
References
Ao T, Deb CR, Khruomo N (2016). Wild edible mushrooms of Nagaland, India: A potential food resource. Journal of Experimental Biology and Agricultural Sciences 4(1):59-65. http://dx.doi.org/10.18006/2015.4(1).59.65
Asan A (2004). Aspergillus, Penicillium and related species reported from Turkey. Mycotaxon 89(1):155-157.
Atlas RM (2004). Handbook of microbiological media. CRC Press LLC, Boca Raton, Florida.
Bamett HL (1965). Illustrated genera of imperfect fungi. Burgess Publishing Company, Minnea Polis.
Bisi-Johnson MA, Obi CL, Ekosse GE (2010). Microbiological and health related perspectives of geophagia: An overview. African Journal of Biotechnology 9(36):5784-5791. https://doi.org/10.5897/AJB2010.000-3307
Bridge P, Spooner B (2001). Soil fungi: Diversity and detection. Plant and Soil 232:147-154. https://doi.org/10.1023/A:1010346305799
Christensen M (1989). A view of fungal ecology. Mycologia 81(1):1-19. https://doi.org/10.1080/00275514.1989.12025620
Domsch KH, Gams W, Anderson T (1980). Compendium of soil fungi. Academic Press, London.
Frisvad JC, Samson RA (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and airborne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49:1-173.
Gilman JC (2001). A manual of soil fungi. Biotech Books, Delhi, India.
Hajiboland R (2017). Environmental and nutritional requirements for tea cultivation. Folia Horticulturae 29(2):199-220. https://doi.org/10.1515/fhort-2017-0019
Jamir T, Ajungla T (2018). Morphological characterization of fungi in tea garden. International Journal of Basic and Applied Research 8(2):296-303.
Karaoglu SA, Ulker S (2006). Isolation, identification and seasonal distribution of soilborne fungi in tea growing areas of Iyidere-Ikizdere vicinity (Rize-Turkey). Journal of Basic Microbiology 46(3):208-218. https://doi.org/10.1002/jobm.200510030
Klich MA (2002). Identification of common Aspergillus species. Central Bureau Voor Schimmel Cultures, Utrecht, Netherlands.
Kumar R, Tapwal A, Pandey S, Borah RK, Borah D, Borgohain J (2013). Macro-fungal diversity and nutrient content of some edible mushrooms of Nagaland, India. Nusantara Bioscience 5 (1):1-7. http://dx.doi.org/10.13057/Nusbiosci/N050101
Nelson PE, Toussoun TA, Marasas WFO (1983). Fusarium species: An illustrated manual for identification. The Pennsylvania State University Press, Pennsylvania, USA.
Pandya ND, Desai PV, Jadhav HP, Sayyed, RZ (2018). Plant growth promoting potential of Aspergillus sp. NPF7, isolated from wheat rhizosphere in South Gujarat, India. Environmental Sustainability 1:245-252. https://doi.org/10.1007/s42398-018-0025-z
Pandey A, Palni LMS, Bisht D (2001). Dominant fungi in the rhizosphere of established tea bushes and their interaction with the dominant bacteria under in situ conditions. Microbiological Research 156(4):377-382. https://doi.org/10.1078/0944-5013-00123
Pitt JI, Hocking AD (2009). Fungi and food spoilage. Springer Dordrecht, Heidelberg, New York.
Raper KB, Thom C (1949). A manual of Penicillia. Williams and Wilkins Co., Baltimore, U.S.A.
Rifai MA (1969). A revision of the genus Trichoderma. In: Mycological Paper No. 116. Commonwealth Mycological Institute, International, Wallingford, UK, pp 1-56.
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHW, … Frisvad, JC (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78:141-173. https://doi.org/10.1016/j.simyco.2014.07.004
Senanayake IC, Rathnayaka AR, Marasinghe DS, Calabon MS, Gentekaki E, Lee HB, ... Xiang MM (2020). Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere 11(1):2678-2754. https://doi.org/10.5943/mycosphere/11/1/20
Siddiquee S (2017). Morphology-based characterization of Trichoderma species. In: Practical handbook of the biology and molecular diversity of Trichoderma species from tropical regions. Springer International Publishing, pp 41-73. https://doi.org/10.1007/978-3-319-64946-7_4
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013). Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587. https://doi.org/10.1186/2193-1801-2-587
Thiep NV, Soytong K, Thi Kim Oanh N, Huy Quang P, Hai Yen P (2019). Research and development of enzymatic producing fungi as biofertilizer for tea and arabica coffee production in Northern Vietnam. International Journal of Agricultural Technology 15(5):797-806.
Waksman SA (1922) A method of counting the number of fungi in the soil. Journal of Bacteriology 7(3):339-341.
Wang H, Hyde KD, Soytong K, Lin F (2008). Fungal diversity on fallen leaves of Ficus in northern Thailand. Journal of Zhejiang University Science 9:835-841.
Wabang T, Ajungla T (2016). Edible, medicinal and red listed monkey head mushroom Hericium erinaceus (Bull.) Pers. from Japfu mountain of Kohima needs immediate protection. Current Botany 7:33-35. http://dx.doi.org/10.19071/cb.2016.v7.3064
Watanabe T (2002). Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC Press, Boca Raton. https://doi.org/10.1201/9781420040821
Wu B, Hussain M, Zhang W, Stadler M, Liu X, Xiang M (2019). Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 10(3):127-140. https://doi.org/110.1080/21501203.2019.1614106
Yadav J, Verma JP, Tiwari KN (2011). Plant growth promoting activities of fungi and their effect on chickpea plant growth. Asian Journal of Biological Sciences 4(3):291-299. https://dx.doi.org/10.3923/ajbs.2011.291.299
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















