Production, purification and characterization of thermostable alpha amylase from Bacillus subtilis Y25 isolated from decaying yam (Dioscorea rotundata) tuber
DOI:
https://doi.org/10.15835/nsb12110521Keywords:
α-amylase; Bacillus subtilis; characterization; optimization; starch; thermostableAbstract
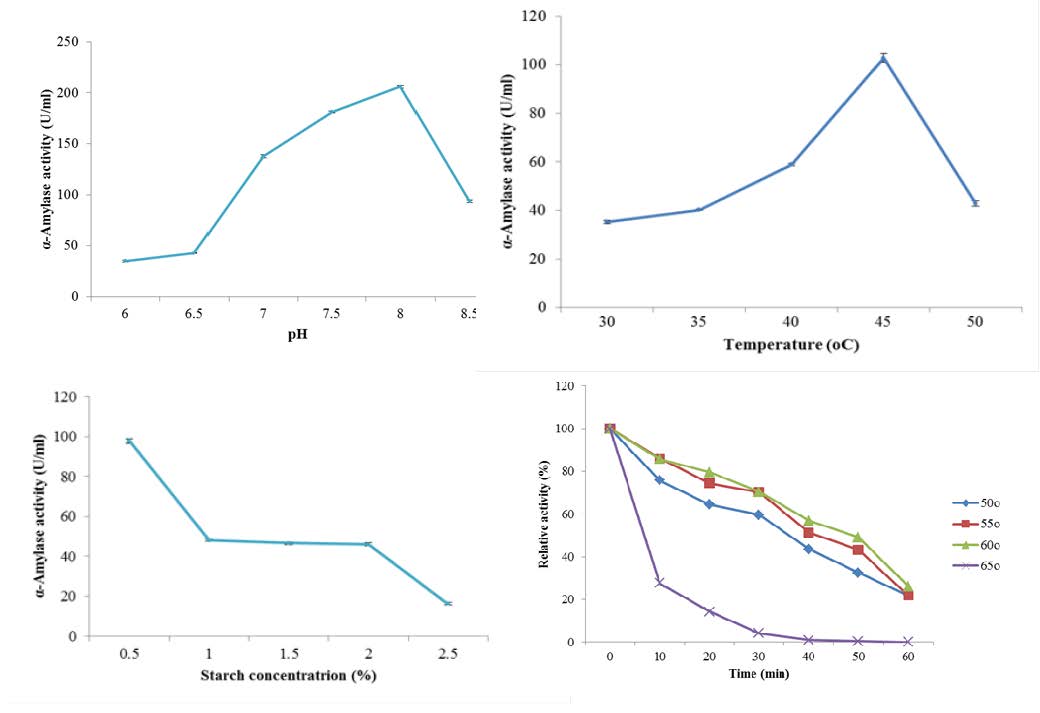
Amylases have wide biotechnological potentials for applications in various industries. An α-amylase-producing bacterium was isolated from deteriorating yam tubers. Molecular characterization using the 16S rRNA gene sequencing was used to confirm the identity of the bacterium as Bacillus subtilis Y25. The effect of some cultural and nutritional factors such as pH, temperature, carbon and nitrogen sources on α-amylase production from the bacterium was determined. Maximum α-amylase production was observed using starch and peptone as carbon and nitrogen sources, respectively, with an initial medium pH of 8.0 and incubation at 45 °C for 36 h. The enzyme was purified by ion exchange chromatography on CM Sepharose CL-6B. The kinetic parameters Km and Vmax of the enzyme, as well as the effect of pH, temperature, metal ions and ethylenediaminetetra acetic acid (EDTA) on the activity of the purified enzyme were studied. The specific activity of the partially purified enzyme was determined to be 15.21 Units/mg protein with a purification fold of 3.80. The molecular weight of the purified enzyme was estimated to be 58.0 kDa. The Vmax and Km values obtained with soluble starch for Bacillus subtilis Y25 α-amylase were 314.10 ± 23.30 Units/mg protein and 53.98 ± 12.03 mg/ml, respectively. The enzyme exhibited optimum activity at a temperature of 60 °C and pH 8.0. The metal ion Ca2+ had no effect on the enzyme at 20 mM concentration, whereas Na+ and Mg2+, as well as EDTA inhibited the enzyme at the same concentration. The characteristics of the α-amylase from Bacillus subtilis Y25 revealed it to be a thermostable and an alkaline metalloenzyme with potential for applications in the detergent and saccharification industries.
Metrics
References
Abdel-Fattah YR, Soliman NA, El-Toukhy NM, El-Gendi H, Ahmed RS (2012). Production, purification and characterization of thermostable α-amilase produced by Bacilluslicheniformis isolate AI20. Journal of Chemistry 2013:1-11. https://doi.org/10.1155/2013/673173
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. Journal of Molecular Biology 215(3):403-410.
https://doi.org/10.1016/S0022-2836(05)80360-2
Anupama A, Jayarama G (2011). Detergent stable, halotolerant α-amylase from Bacillus Aquimaris VITP4 exibits reversible unfolding. International Journal of Applied Biology and Pharmaceutical Technology 2(2):366-376.
Asgher M, Asad MJ, Rahman SU, Legge RL (2007). A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. Journal of Food Engineering 79:950-955. https://doi.org/10.1016/j.jfoodeng.2005.12.053
Ayansina ADV, Adelaja AO, Mohammed SS (2017). Characterization of amylase from some Aspergillus and Bacillus species associated with cassava waste peels. Advances in Microbiology 7:280-292.
Azad MA, Bae JH, Kim JS, Lim JK, Song KS, Shin BS, Kim HR (2009). Isolation and characterization of a novel thermostable alpha-amylase from Korean pine seeds. Nature Biotechnology 26:143-149. https://dx.doi.org/10.1016/j.nbt.2009.09.006
Bakare MK, Omoboye OO, Adewale IO, Awojobi KO, Oyedeji O (2014). Purification and characterization of thermostable alpha-amylase by Bacillus licheniformis RD 24 isolated from decayed refuse at Obafemi Awolowo University Campus, Ile-Ife, Nigeria. Frontiers of Biological and Life Sciences 2(4):74-84. http://dx.doi.10.12966/fbls.12.03.2014
Benjamin S, Smitha RB, Jisha VN, Pradeep S, Sajith S, Sreedevi S, Priji P, Unni KN, Sarath Josh MK (2013). A monograph on amylases from Bacillus spp. Advances in Biosciences and Biotechnology 4:227-241. doi:10.4236/abb.2013.42032
Bijttebier A, Goesaert H, Delcour JA (2008). Amylase action pattern on starch polymers. Biologia 63(6):989-999.
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72:248-254 https://doi.org/10.1016/0003-2697(76)90527-3
Castro AM, Carvalho DF, Freire DMG, Castilho LR (2010). Economic analysis of the production of amylases and other hydrolases by Aspergillus awamori in solid-state fermentation of babassu cake. Enzyme Research 2010:1-9. http://dx.doi.org/10.4061/2010/576872
Das S, Singh S, Sharma V, Soni ML (2011). Biotechnological applications of industrially important amylase enzyme. International Journal of Pharma and BioSciences 2(1):486-496.
Deb P, Talukdar SA, Mohsina K, Sarker PK, Sayem SA (2013). Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. Springerplus 2:154. http://dx.doi.org/10.1186/2193-1801-2-154.
Demirkan E, Sevgi T, Baskurt M (2017). Optimization of physical factors affecting the production of the α-amylase from a newly isolated Bacillus sp. M10 strain. Karaelmas Fen ve Muhendislik Dergisi 7(1):23-30. http://dx.doi.org/10.7212%2Fzkufbd.v1i1.458
El-Helow ER (2001). Identification and molecular characterization of a novel Bacillus strain capable of degrading tween-80. FEMS Microbiology Letters 196(2):119-122. https://doi.org/10.1111/j.1574-6968.2001.tb10551.x
El-Kady EM, Asker MS, Hassanein MS, Elmansy EA, El-Beih FM (2017). Optimization, production and partial purification of thermostable α-amylase produced by marine bacterium Bacillus sp. NRC12017. International Journal of Pharmaceutical and Clinical Research 9(8):558-570. https://dx.doi.org/10.25258/ijpcr.v9i08.9581
Elmansy EA, Asker MS, El-Kady EM, Hassanein SM, El-Beih FM (2018). Production and optimization of α-amylase from thermo-halophilic bacteria isolated from different local marine environments. Bulletin of the National Research Centre (2018) 42:31. http://dx.doi.org/101186/s42269-018-0033-2
Femi-Ola TO, Olowe BM (2011). Characterization of alpha amylase from Bacillus subtilis BS5 isolated from Amitermes evuncifer Silvestri. Research Journal of Microbiology 6:140-146. http://dx.doi.org/10.3923/jm.2011.140.146
Goes AP, Sheppard JD (1999). Effect of surfactants on α-amylase production in a solid substrate fermentation process. Journal of Chemical Technology and Biotechnology 74:709-712.
Gangadharan D, Sivaramakrishnan S, Namboothiri KM, Pandey A (2006). Solid culturing of Bacillus amyloliquefaciens for α-amylase production. Food Technology and Biotechnology 44(2):269-274.
Gomes I, Gomes J, Steiner W (2003). Highly thermostable amylase and pullulanase of the extreme thermophilic eubacterium Rhodothermus marinus: production and partial characterization. Bioresources and Technology 90:207-214.
Gupta R, Giras P, Mohapatra H, Goswami YK (2003). Microbial α-amylase: a biotechnological perspective. Process Biochemistry 38(11):1599-1616.
Hmidet N, Bayoudh A, Berrin JG, Kanoun S (2008). Purification and biochemical characterization of a novel α-amylase from Bacillus licheniformis NH1: Cloning, nucleotide sequence and expression of amyN gene in Escherichia coli. Process Biochemistry 43:499-510.
Khan JA, Yadav SK (2011). Production of α-amylase by Aspergillus niger using cheaper substrates employing solid state fermentation. International Journal of Plant, Animal and Environmental Science 1(3):100-108.
Konsoula Z, Liakopoulou-Kyriakides M (2004). Hydrolysis of starches by the action of an alpha-amylase from Bacillus subtilis. Process Biochemistry 39(11):1745-1749.
Konsoula Z, Liakopoulou-Kyriakides M (2007). Coproduction of alpha-amylase and beta-galactosidase by Bacillus subtilis in complex organic substrates. Bioresources Technology 98:150-157.
Lin LL, Chyau C, Hsu WH (1998). Production and properties of a raw starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Nature 6:68-74.
Liu Y, Lu F, Li Y, Yin X, Wang Y (2008). Acid stabilization of Bacillus licheniformis alpha amylase through mutations. Applied Microbiology and Biotechnology 45:259-267.
Malhotra R, Noorwez S, Satyanarayana T (2000). Production and partial characterization of thermostable and calcium independent α-amylase of an extreme thermophile Bacillus thermooleovorans NP54. Letters in Applied Microbiology 31:378-384.
Matthew JJ, Vazhacharickal PJ, Sajeshkumar NK, John NK (2016). Comparative study of the activity of amylase produced by Aspergillus niger through solid state fermentation (SSF) using various starchy materials. Indian Journal of Plant Science 5:79-90.
Mitidieri S, Souza MAH, Schrank A, Vainstein MH (2006). Enzymatic detergent formulation containing amylase from Aspergillus niger. A comparative study with commercial detergent formulations. Bioresources Technology 97:1217-1224.
Mohammed MAA-Z, Shivayogeeshwar N, Gurumurthy DM, Rajeshwara AN (2011). Identification and characterization of novel halophilic Bacillus cereus Ms6: a source for extra cellular α-amylase. Advances in Environmental Biology 5(5):992-999.
Mukhtar H, Ikram UH (2012). Concomitant production of two proteases and alpha-amylase by a novel strain of Bacillus subtilis in a microprocessor-controlled bioreactor. Brazilian Journal of Microbiology 43:1072-1079.
NandLal Jyoti J, Sachan P (2016). Optimization of nitrogen source(s) for the growth and amylase production from Bacillus licheniformis JAR-26 under submerged fermentation. Indian Journal of Biology 3(2):127-132.
Omemu AM, Akpan I, Bankole MO, Teniola OD (2005). Hydrolysis of raw tuber starches by amylase of Aspergillus niger AM 07 isolated from soil. African Journal of Biotechnology 4:19-25.
Omoboye OO, Bakare MK, Adewale IO, Oyedeji O (2014). Molecular identification and amylolytic potential of a thermophilic bacteria species from refuse dump in Ile-Ife, Nigeria. International Journal of Biological Research 2(2):134-139.
Ozdemir S, Matpan F, Guven K, Baysal Z (2011). Production and characterisation of partially purified extracellular thermostable α-amylase by Bacillus subtilis in submerged fermentation (SmF). Preparative Biochemistry and Biotechnology 41:365-381.
Ozdemir S, Fincan SA, Karakaya A, Enez B (2018). A novel raw starch hydrolysing thermostable α-amylase produced by newly isolated Bacillus mojavensis SO-10: purification, characterization and usage in starch industries. Brazilian Archives of Biology and Technology 61: e18160399. http://dx.doi.org/10.1590/1678-4324-2018160399
Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R (2000). Advances in microbial amylases. Biotechnology and Applied Biochemistry 31:135-152.
Prakash B, Vidyasagar M, Madhukumar MS, Muralikrishna G, Sreeramulu K (2009). Production, purification and characterization of the extremely halotolerant, thermostable and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochemistry 44:210-215. https://doi.org/10.1016/j.procbio.2008.10.013
Reddy NS, Nimmagada A, Sambasiva Rao KRS (2003). An overview of the microbial α-amylase family. African Journal of Biotechnology 2:645-648.
Saxena L, Iyer BK, Ananthanarayan L (2007). Tree phase partitioning as a novel method for purification of ragi (Eleusine coracana) bifunctional amylase/protease inhibitor. Process Biochemistry 42:491-495.
Shalini S, Solanki MK (2014). Optimization of thermostable alpha-amylase production via mix agricultural-residues and Bacillus amyloliquefaciens. Notulae Scientia Biologicae 6(1):105-111.
Singh P, Gupta P, Singh R, Sharma R (2012). Factors affecting alpha amylase production on submerged fermentation by Bacillus sp. International Journal of Pharmaceutical and Life Sciences 3(12):2243-2246.
Singh V, Sharma R, Sharma P (2015). Isolation, screening and optimization of amylase producing Bacillus sp. from soil. Asian Pacific Journal of Health Science 2(3):86-93.
Singh AM, Latha BV, Chethankumar M, Kumar BYS (2016). A comparative study on fungal (Aspergillus niger) amylase and elephant foot yam (Amorphophallus campanulatus) amylase with yam starch as substrate. International Journal of Applied Research 2:1006-1010.
Sivaramakrishnan S, Gangadharan D, Nampoothiri M (2006). α-Amylases from microbial sources. Food Technology and Biotechnology 44:173-184.
Somogyi M (1952). Notes on sugar determination. Journal of Biological Chemistry 194:19-21.
Smitha RB, Benjamin S, Prakashkumar R (2019). Fermentation strategies for the production of α-amylase and δ-endotoxin from Bacillus thuringiensis subsp. Kurstaki. Avid Science Monograph Series 1(192):37.
Souza PM, Magalhaes PO (2010). Applications of microbial amylases in industry – a review. Brazilian Journal of Microbiology 41:850-861.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013). MEGA 6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30(12):2725-2729. http://dx.doi.10.1093/molbev/mst197
Wanderley KJ, Torres FA, Moraes LM, Ulhoa CJ (2004). Biochemical characterization of alpha-amylase from the yeast Cryptococcus flavus. FEMS Microbiology Letters 231:165-169.
Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G (2014). Purification and characterization of a novel α-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochemistry 49:47-53.
Yang H, Liu L, Li J, Du G, Chen J (2011). Heterologous expression, biochemical characterization, and overproduction of alkaline α-amylase from Bacillus alcalophilus in Bacillus subtilis. Microbial Cell Factories 10:77. http://dx.doi:10.1186/1475-2859-10-77
Zeng J, Gao X, Dai Z, Tang B, Tang XF (2014). Effects of metal ions on stability and activity of hyperthermophilic pyrolysin and further stabilization of this enzyme by modification of a Ca2+-binding site. Applied and Environmental Microbiology 80(9):2763-2772. http://dx.doi:10.1128/AEM.00006-14
Downloads
Published
How to Cite
Issue
Section
License
Papers published in Notulae Scientia Biologicae are Open-Access, distributed under the terms and conditions of the Creative Commons Attribution License.
© Articles by the authors; licensee SMTCT, Cluj-Napoca, Romania. The journal allows the author(s) to hold the copyright/to retain publishing rights without restriction.
License:
Open Access Journal - the journal offers free, immediate, and unrestricted access to peer-reviewed research and scholarly work, due SMTCT supports to increase the visibility, accessibility and reputation of the researchers, regardless of geography and their budgets. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author.













.png)















